Intramolecular macrolactonization, photophysical and biological studies of new class of polycyclic pyrrole derivatives†
Abstract
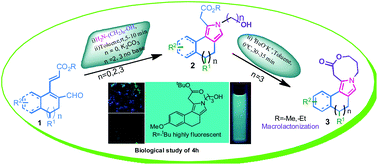
Herein, we report an efficient synthesis of N-substituted pyrrole derivatives and their application to construct macrocyclic oxazocinone via a two-component coupling reaction followed by base mediated intramolecular cyclization. This methodology provides an easy two-step approach to constitute a library of fused pyrrolo-oxazocinone derivatives in good yields under mild reaction conditions. The present methodology offers an easy access to the synthesis of a library of fluorescent pyrole derivatives. Among them, tert-butyl 2-(2-(3-hydroxypropyl)-7-methoxy-4,5-dihydro-2H-benzo[e]isoindol-1-yl)acetate has been employed in bio-analytical imaging which shows efficient cellular internalization along with no obvious cellular toxicity.


 Please wait while we load your content...
Please wait while we load your content...