Total synthesis and biological activity of dolastatin 16†
Abstract
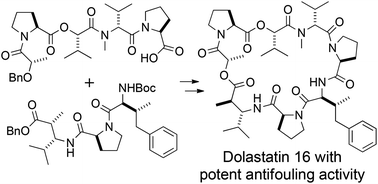
The total synthesis of dolastatin 16, a macrocyclic depsipeptide first isolated from the sea hare Dolabella auricularia as a potential antineoplastic metabolite by Pettit et al., was achieved in a convergent manner. Dolastatin 16 was reported by Tan to exhibit strong antifouling activity, and thus shows promise for inhibiting the attachment of marine benthic organisms such as Amphibalanus amphitrite to ships and submerged artificial structures. Therefore, dolastatin 16 is a potential compound for a new, environmentally friendly antifouling material to replace banned tributyltin-based antifouling paints. The synthesis of dolastatin 16 involved the use of prolinol to prevent formation of a diketopiperazine composed of L-proline and N-methyl-D-valine during peptide coupling. This strategy for the elongation of peptide chains allowed the efficient and scalable synthesis of one segment, which was subsequently coupled with a second segment and cyclized to form the macrocyclic framework of dolastatin 16. The synthetic dolastatin 16 exhibited potent antifouling activity similar to that of natural dolastatin 16 toward cypris larvae of Amphibalanus amphitrite.


 Please wait while we load your content...
Please wait while we load your content...