Cooperativity of axial and centre chirality in the biaryl disulfoxide/Rh(i)-catalysed asymmetric 1,4-addition of arylboronic aids to 2-cyclohexenone: a DFT study†
Abstract
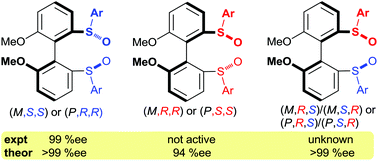
Atropisomeric biaryl disulfoxides contain two independent chiral elements. Previously, the (M,S,S)-diastereomer showed very high catalytic activity and selectivity in the Rh-catalyzed asymmetric 1,4-addition of arylboronic acids to α,β-enones whereas the (M,R,R) counterpart – none. Herein, DFT computations on the key transmetallation (turnover-determining) and carborhodation (enantioselectivity-determining) steps of the catalytic cycle show that the (M,S,S)-ligand gives rise to lower reaction barriers for these elementary steps. However, the barriers for the (M,R,R)-ligand are not sufficiently high to explain the lack of reactivity. Hence, this phenomenon is most likely due to the failure of catalyst formation from the ligand and the dimeric Rh precatalyst complex. The hitherto unknown (M,S,R)-ligand shows predicted enantioselectivity similar to the (M,S,S)-ligand as a consequence of lower reaction barriers associated with those isomers whose key features resemble the (M,S,S)-ligand.


 Please wait while we load your content...
Please wait while we load your content...