Catalytic asymmetric Tamura cycloadditions involving nitroalkenes†
Abstract
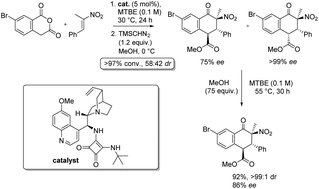
The first examples of asymmetric Tamura cycloaddition reactions involving singly activated alkenes are reported. Homophthalic anhydride reacts with α-methyl nitrosytrenes in the presence of an alkaloid-based catalyst to generate fused bicyclic aromatic ketone products with three new stereocentres (which are susceptible to subsequent equilibration) in 12–99% ee. An unusual equilibration process which occurs in methanolic medium in the absence of a recognisable base via proton transfer at the α-carbon of an ester was investigated experimentally and computationally.


 Please wait while we load your content...
Please wait while we load your content...