Diastereoselective synthesis of trifluoromethylated 1,3-dioxanes by intramolecular oxa-Michael reaction†
Abstract
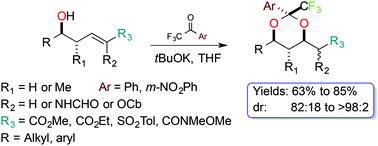
A highly diastereoselective synthesis of trifluoromethylated 1,3-dioxanes is described. The reaction proceeds by an addition/oxa-Michael sequence and works efficiently under mild reaction conditions, with a good substrate scope and acceptable to good yields.


 Please wait while we load your content...
Please wait while we load your content...