The 5-chlorouracil:7-deazaadenine base pair as an alternative to the dT:dA base pair†
Abstract
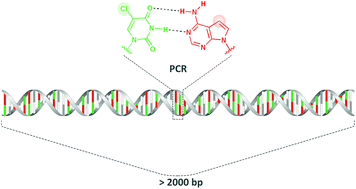
5-Chloro-2′-deoxyuridine as a possible component of a chemically modified genome has been discussed in terms of its influence on duplex stability and DNA polymerase incorporation properties. The search for its counterpart among different deoxyadenosine analogs (7-deaza-, 8-aza- and 8-aza-7-deaza-2′-deoxyadenosines) showed that the stable duplex formation as well as the synthesis of long constructs, more than 2 kb, were successful with the 5-chloro-2′-deoxyuridine and 7-deaza-2′-deoxyadenosine combination and with Taq DNA polymerase.

 Please wait while we load your content...
Please wait while we load your content...