Shining new light on ancient drugs: preparation and subcellular localisation of novel fluorescent analogues of Cinchona alkaloids in intraerythrocytic Plasmodium falciparum†
Abstract
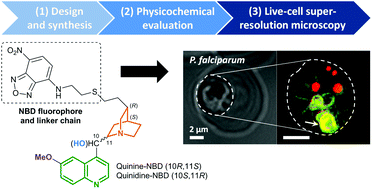
Fluorescent derivatives of the archetypal antimalarial quinine and its diastereomer, quinidine, suitable for cellular imaging have been synthesised by attaching the small extrinsic fluorophore, NBD. Interactions of these derivatives with ferriprotoporphyrin IX were evaluated to verify that insights generated by live-cell imaging were relevant to the parent molecules. These analogues are shown by confocal and super-resolution microscopy to accumulate selectively in Plasmodium falciparum. Localisation to the region corresponding to the digestive vacuole supports the putative primary role of these alkaloids as haemozoin inhibitors. Quantitative analysis revealed minimal accumulation within the nucleus, rejecting the disruption of DNA replication as a possible mode of action. While extensive localisation to phospholipid structures and associated organelles was observed, the analogues did not show evidence of association with neutral lipid bodies.
- This article is part of the themed collection: #RSCPoster Conference

 Please wait while we load your content...
Please wait while we load your content...