Bicyclic enol cyclocarbamates inhibit penicillin-binding proteins†
Abstract
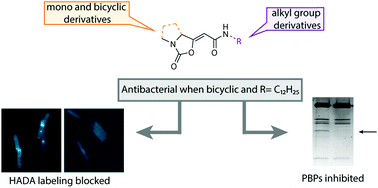
Natural products form attractive leads for the development of chemical probes and drugs. The antibacterial lipopeptide Brabantamide A contains an unusual enol cyclocarbamate and we used this scaffold as inspiration for the synthesis of a panel of enol cyclocarbamate containing compounds. By equipping the scaffold with different groups, we identified structural features that are essential for antibacterial activity. Some of the derivatives block incorporation of hydroxycoumarin carboxylic acid-amino D-alanine into the newly synthesized peptidoglycan. Activity-based protein-profiling experiments revealed that the enol carbamates inhibit a specific subset of penicillin-binding proteins in B. subtilis and S. pneumoniae.


 Please wait while we load your content...
Please wait while we load your content...