Enhanced ionic conductivity in electroceramics by nanoscale enrichment of grain boundaries with high solute concentration†
Abstract
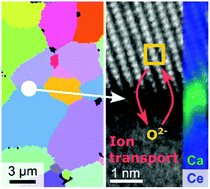
The enhancement of oxygen ionic conductivity by over two orders of magnitude in an electroceramic oxide is explicitly shown to result from nanoscale enrichment of a grain boundary layer or complexion with high solute concentration. A series of CaxCe1−xO2−δ polycrystalline oxides with fluorite structure and varying nominal Ca2+ solute concentration elucidates how local grain boundary composition, rather than structural grain boundary character, primarily regulates ionic conductivity. A correlation between high grain boundary solute concentration above ∼40 mol%, and four orders of magnitude increase in grain boundary conductivity is explicitly shown. A correlated experimental approach provides unique insights into fundamental grain boundary science, and highlights how novel aspects of nanoscale grain boundary design may be employed to control ion transport properties in electroceramics.


 Please wait while we load your content...
Please wait while we load your content...