Self-assembly of water-soluble silver nanoclusters: superstructure formation and morphological evolution†
Abstract
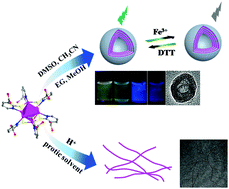
Supramolecular self-assembly, based on non-covalent interactions, has been employed as an efficient approach to obtain various functional materials from nanometer-sized building blocks, in particular, [Ag6(mna)6]6−, mna = mercaptonicotinate (Ag6-NC). A challenging issue is how to modulate the self-assembly process through regulating the relationship between building blocks and solvents. Herein, we report the controlled self-assembly of hexanuclear silver nanoclusters into robust multilayer vesicles in different solvents, DMSO, CH3CN, EG and MeOH. Their unique luminescence enables them to be bifunctional probes to sense Fe3+ and DL-dithiothreitol (DTT). By protonating the Ag6-NC to Ag6-H-NC using hydrochloric acid (HCl), the multilayer vesicles survive in aprotic solvents, DMSO and CH3CN, but are transformed to nanowires in protic solvents, water, EG and MeOH. Our results demonstrated that the solvent-bridged H-bond plays a key role in the evolution of the morphologies from vesicles to nanowires. Moreover, the nanowires could further hierarchically self-assemble in water into hydrogels with high water content (99.5%), and with remarkable mechanical strength and self-healing properties. This study introduces a robust cluster-based building block in a supramolecular self-assembly system and reveals the significance of aprotic and protic solvents for the modulation of the morphologies of cluster-based aggregates.


 Please wait while we load your content...
Please wait while we load your content...