On-surface synthesis of heptacene and its interaction with a metal surface†
Abstract
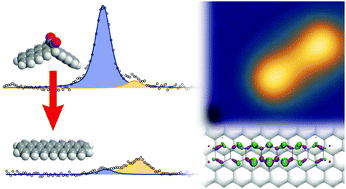
Heptacene was generated by surface-assisted didecarbonylation of an α-diketone precursor on a Ag(111) surface. Monitoring of the surface reaction and characterization of the adsorbed heptacene was performed with scanning tunneling microscopy (STM), X-ray photoelectron spectroscopy (XPS), near-edge X-ray absorption fine structure (NEXAFS) spectroscopy, and density functional theory (DFT) calculations. The surface-assisted formation of heptacene occurs around 460 K. Both the heptacene and the precursor molecules are oriented along the high-symmetry directions of the (111) surface and their molecular π systems face towards the substrate. The interaction with the Ag(111) substrate is not laterally uniform, but appears to be strongest on the central part of the molecule, in line with the expectations from Clar's rule. In the STM images, heptacene shows a dumbbell shape, which may correspond to the substantial out-of-plane deformations of heptacene on Ag(111). As revealed by DFT, the center of the molecule is closer to the surface than the outer parts. In addition, the inner rings are most affected by charge redistribution between surface and molecule. Heptacene acts as an acceptor and receives a negative charge of −0.6e from the Ag(111) surface. Since vacuum-sublimable α-diketone precursors for even larger acenes are available, the approach is promising for the on-surface synthesis of higher acene homologues such as octacene and nonacene.


 Please wait while we load your content...
Please wait while we load your content...