Amorphous boron nanorod as an anode material for lithium-ion batteries at room temperature†
Abstract
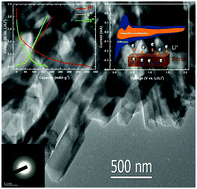
We report an amorphous boron nanorod anode material for lithium-ion batteries prepared through smelting non-toxic boron oxide in liquid lithium. Boron in theory can provide capacity as high as 3099 mA h g−1 by alloying with Li to form B4Li5. However, experimental studies of the boron anode have been rarely reported for room temperature lithium-ion batteries. Among the reported studies the electrochemical activity and cycling performance of the bulk crystalline boron anode material are poor at room temperature. In this work, we utilized an amorphous nanostructured one-dimensional (1D) boron material aiming at improving the electrochemical reactivity between boron and lithium ions at room temperature. The amorphous boron nanorod anode exhibited, at room temperature, a reversible capacity of 170 mA h g−1 at a current rate of 10 mA g−1 between 0.01 and 2 V. The anode also demonstrated good rate capability and cycling stability. The lithium storage mechanism was investigated by both sweep voltammetry measurements and galvanostatic intermittent titration techniques (GITTs). The sweep voltammetric analysis suggested that the contributions from lithium ion diffusion into boron and the capacitive process to the overall lithium charge storage are 57% and 43%, respectively. The results from GITT indicated that the discharge capacity at higher potentials (>∼0.2 V vs. Li/Li+) could be ascribed to a capacitive process and at lower potentials (<∼0.2 V vs. Li/Li+) to diffusion-controlled alloying reactions. Solid state nuclear magnetic resonance (NMR) measurement further confirmed that the capacity is from electrochemical reactions between lithium ions and the amorphous boron nanorod. This work provides new insights into designing nanostructured boron materials for lithium-ion batteries.


 Please wait while we load your content...
Please wait while we load your content...