Towards highly active Pd/CeO2 for alkene hydrogenation by tuning Pd dispersion and surface properties of the catalysts†
Abstract
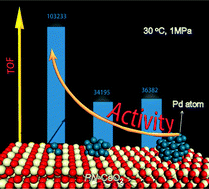
Extensive applications of noble metals as heterogeneous catalysts are limited by their global reserve scarcity and exorbitant price. Identifying the intrinsic active nature of a catalyst benefits the designing of catalysts with trace amounts of noble metals; these catalysts display better or comparable overall catalytic efficiency than their heavily loaded counterparts. Herein, systematic studies on Pd dispersion and surface properties of a series of Pd/CeO2 catalysts for styrene hydrogenation showed that high Pd dispersion and surface abundant defects of the catalysts are essential to realize superior activity. Highly dispersed subnanometric Pd clusters on porous nanorods of ceria with a large surface Ce3+ fraction of 27.4%, high Pd dispersion of 73.6%, and low Pd loading of 0.081 wt% delivered a very large turnover frequency of 103 233 h−1 based on each exposed Pd atom for styrene hydrogenation at 1.0 MPa H2 and 30 °C. Experimental data, kinetic analysis, and density functional theory calculations revealed that the highly dispersed Pd shows a low affinity for styrene and provides more exposed Pd sites for hydrogen activation. The surface abundant defects (oxygen vacancy) of Pd/CeO2 catalysts can enrich the electron density of Pd, improve its capability for H2 dissociation and lower the affinity of styrene for Pd.


 Please wait while we load your content...
Please wait while we load your content...