Effect of ammonia on chemical vapour deposition and carbon nanotube nucleation mechanisms†
Abstract
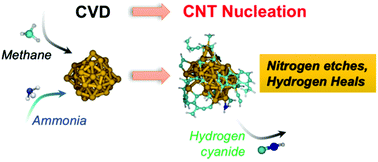
Chemical vapour deposition (CVD) growth of carbon nanotubes is currently the most viable method for commercial-scale nanotube production. However, controlling the ‘chirality’, or helicity, of carbon nanotubes during CVD growth remains a challenge. Recent studies have shown that adding chemical ‘etchants’, such as ammonia and water, to the feedstock gas can alter the diameter and chirality of nanotubes produced with CVD. To date, this strategy for chirality control remains sub-optimal, since we have a poor understanding of how these etchants change the CVD and nucleation mechanisms. Here, we show how ammonia alters the mechanism of methane CVD and single-walled carbon nanotube nucleation on iron catalysts, using quantum chemical molecular dynamics simulations. Our simulations reveal that ammonia is selectively activated by the catalyst, and this enables ammonia to play a dual role during methane CVD. Following activation, ammonia nitrogen removes carbon from the catalyst surface exclusively via the production of hydrogen (iso)cyanide, thus impeding the growth of extended carbon chains. Simultaneously, ammonia hydrogen passivates carbon dangling bonds, which impedes nanotube nucleation and promotes defect healing. Combined, these effects lead to slower, more controllable nucleation and growth kinetics.


 Please wait while we load your content...
Please wait while we load your content...