Catalytic macrolactonizations for natural product synthesis
Abstract
Covering: 2000 to 2017
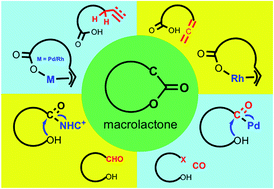
Macrolactones are privileged structural motifs in many functional molecules, particularly natural products and pharmaceutical molecules. They are commonly synthesized from the corresponding seco acids with various stoichiometric activating reagents to promote the formation of the macrocycle. Advances in new methods and strategies for synthesizing macrolactones have been made over the years to improve the overall synthetic efficiency and economy. This highlight focuses on the recent developments of catalytic macrolactonization methods and strategies without the use of seco acids and their applications in natural product total synthesis. In particular, catalytic C–H macrolactonization, enantioselective Rh-catalyzed redox-neutral allene-acid cyclization, catalytic carbonylative macrolactonization, and NHC-catalyzed oxidative macrolactonization are highlighted.


 Please wait while we load your content...
Please wait while we load your content...