NHC catalyzed enantioselective Coates-Claisen rearrangement: a rapid access to the dihydropyran core for oleuropein based secoiridoids†
Abstract
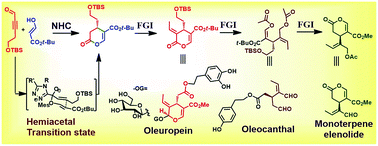
We present the short synthesis of the suitably functionalized enantioselective dihydropyran core of secoiridoids using an N-heterocyclic carbene (NHC) catalyzed Coates-Claisen rearrangement mechanism. The key steps of the synthesis are (i) the highly enantioselective NHC catalyzed Coates-Claisen rearrangement for the dihydropyran core, (ii) the assembly of the target dihydropyran core structure of oleuropein from a highly diastereoselective exocyclic trans alkene, and (iii) the highly stereoselective assembly of a monoterpene elenolide core structure.


 Please wait while we load your content...
Please wait while we load your content...