C3-Symmetric star shaped donor–acceptor truxenes: synthesis and photophysical, electrochemical and computational studies†
Abstract
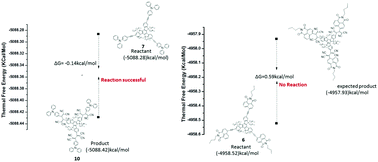
This manuscript reports the design and synthesis of C3-symmetric star shaped donor and acceptor substituted truxenes 6, 7, 10 and 11 using Pd-catalyzed Sonogashira cross-coupling and [2+2] cycloaddition–retroelectrocyclization reactions. Their photophysical, electrochemical and computational studies were explored, which indicate strong donor–acceptor interactions and effective tuning of the HOMO–LUMO gap. The computational studies reveal that the TCNE and TCNQ substituted truxenes 10 and 11 exhibit lower HOMO–LUMO gaps compared to truxenes 6 and 7. The reaction pathway of [2+2] cycloaddition–retroelectrocyclization was studied using computational calculations, which reveal that the donor substituted truxene 7 is favourable for cycloaddition–retroelectrocyclization reactions, whereas acceptor substituted truxene 6 is not favourable.


 Please wait while we load your content...
Please wait while we load your content...