Remarkably selective NH4+ binding and fluorescence sensing by tripodal tris(pyrazolyl) receptors derived from 1,3,5-triethylbenzene: structural and theoretical insights on the role of ion pairing†
Abstract
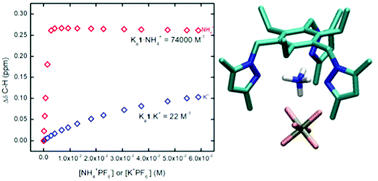
A fluorescent sensor for NH4+ based on 1,3,5-triethylbenzene shows remarkable binding and sensing selectivity for NH4+vs. K+. Fluorescence and NMR titrations reveal surprising differences in the sensing properties and binding constants of tris-(3,5-dimethyl)pyrazole 1vs. tris(3,5-diphenyl)pyrazole 2. The roles of ion pairing and solvation are revealed by X-ray and DFT studies.


 Please wait while we load your content...
Please wait while we load your content...