Studying the fluorescence conversion in organic charge transfer cocrystals of chalcone derivatives and TCNB†
Abstract
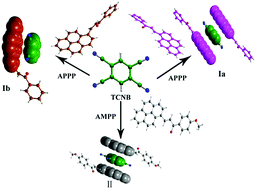
Four binary charge-transfer cocrystals (Ia, Ib, II and III) were engineered by a solvent evaporation method, involving 1-phenyl-3-(1-pyrenyl)prop-2-en-1-one (PPPO, I), 3-(1-pyrenyl)-1-(4-methoxyphenyl)prop-2-en-1-one (PMPPO, II) and 3-(1-pyrenyl)-1-(4-chlorophenyl)prop-2-en-1-one (PCPPO, III) as electron donors (D) and 1,2,4,5-benzenetetracarbonitrile (TCNB) as an electron acceptor (A). The structures and optical properties of these complexes were investigated by single crystal X-ray diffraction (SCXRD), powder X-ray diffraction (PXRD), UV-Vis absorption spectroscopy and fluorescence spectroscopy. CrystalExplorer was also used to analyze the surface force of the crystals. The studies showed that the fluorescence of all of these cocrystals exhibits a significant red-shift, and this phenomenon is caused by charge-transfer (CT) interactions between the donor and acceptor. In addition, the differences in fluorescence to the pristine molecules may provide these CT cocrystals with a wide range of applications in the prospective field of opto-electronic materials.


 Please wait while we load your content...
Please wait while we load your content...