Efficiently synthesized Co doped Cu3TeO6 accounted for its anomalous behaviour in electronic properties†
Abstract
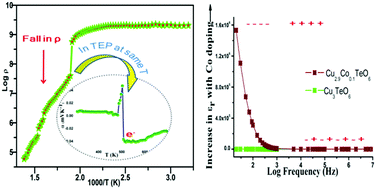
Synthesis of Cu3−xCoxTeO6 (x = 0, 0.1, 0.3 or 0.5) is carried out using a wet chemical route and characterized using various instrumental techniques. The cubic crystal structure of these compounds is confirmed using an X-ray diffraction method. Morphological observations are made using scanning electron and transmission electron microscopy (SEM-TEM) techniques and the stoichiometries of the elemental composition of the compounds are investigated using SEM-energy dispersive X-ray spectroscopy (SEM-EDS) analysis. X-ray photoelectron spectroscopy measurements are carried out and studied in detail in order to examine the valence state of the elements present. A direct current electrical resistivity study and a thermoelectric power study give a clear picture of the electrical transition occurring, which is also evident from the differential scanning calorimetry studies. Compounds show a magnetic transition from an anti-ferromagnetic type to a paramagnetic type at the material's Néel temperature. Enhancement in the dielectric properties of copper tellurate (Cu3TeO6) on doping with cobalt (Co) is observed when studied under frequency domain. In general, this research shows an efficient synthetic route and a detailed study of Co doped copper tellurates, which could provide new opportunities for researchers to explore its applications in the field of electronics.


 Please wait while we load your content...
Please wait while we load your content...