A colorimetric biosensor based on guanidinium recognition for the assay of protein tyrosine phosphatase 1B and its inhibitors
Abstract
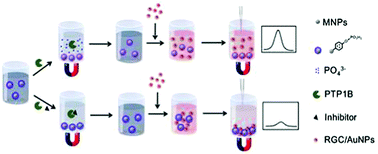
In this study, a colorimetric biosensor was established for the detection of protein tyrosine phosphatase 1B (PTP1B) and its inhibitor based on the guanidinium recognition assisted coordination of arginine-glycine-cysteine (RGC) modified gold nanoparticles (RGC/AuNPs) with 4-aminophenyl phosphate-functionalized Fe3O4 magnetic nanoparticles (MNPs/APP). PTP1B can catalyze the cleavage of the phosphate ester bond and the departure of the phosphate acid group from the surface of MNPs/APP, resulting in the loss of the corresponding coordination reactivity. Upon the addition of a solution of RGC/AuNPs, RGC/AuNPs remained in the supernatant solution after magnetic separation and a high absorbance value was observed. Thus, a simple colorimetric biosensor for PTP1B assay was developed. Under the optimized experimental conditions, PTP1B was detected within a linear range of 0.002 U mL−1 to 0.08 U mL−1 with the lowest detection limit of 0.0013 U mL−1. Moreover, using this proposed biosensor, the inhibition effect of betulinic acid and 6-chloro-3-formyl-7-methylchromone on PTP1B is determined with IC50 values of 9 μM and 15 μM, respectively. Therefore, this new biosensor not only has great potential for PTP1B analysis but also for the detection of its inhibitors.


 Please wait while we load your content...
Please wait while we load your content...