High-performance ambipolar responses to oxidizing NO2 and reducing NH3 based on the self-assembled film of an amphiphilic tris(phthalocyaninato) europium complex
Abstract
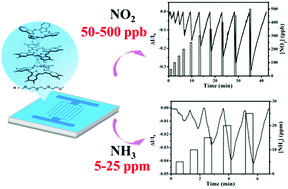
A heteroleptic amphiphilic tris(phthalocyaninato) europium triple-decker complex, (Pc)Eu{Pc[(OC2H4)3OCH3]8}Eu{Pc[(OC2H4)3OCH3]8} (1), has been designed and synthesized successfully. The hydrophilic polyoxyethylene substituents attached onto the periphery of two phthalocyanine rings in the sandwich-type phthalocyaninato triple-decker not only increase the solubility and improve the film-forming ability, but also importantly ensure the suitable HOMO and LUMO energy levels and thus successfully realize amphiphilic ambipolar organic semiconductors. The solution-processed thin film of the complex is prepared by a simple and low-cost quasi-Langmuir–Shäfer (QLS) method. Importantly, within the dynamic exposure period of 30 s, highly sensitive, stable, reproducible n-type response to electron-accepting gas NO2 in the range of 50–500 ppb and p-type response to electron-donating gas NH3 in 5–25 ppm range, have been first revealed, based on the QLS film of 1 at room temperature, depending on the optimized molecular packing in J-aggregation mode with a large specific surface area and good film conductivity. Furthermore, the responses of the QLS film are all linearly correlated with either NO2 or NH3 with good sensitivity of 0.06% ppb−1 and 0.17% ppm−1, respectively, indicating the great potential of phthalocyanine-based rare earth triple-decker complexes in the field of chemical sensors. The present result provides a new strategy to obtain high-performance room-temperature gas sensors by the molecular design combined with a low-cost solution-based method.


 Please wait while we load your content...
Please wait while we load your content...