Synthesis, characterisation and self-assembly behaviour of emissive surfactant–ruthenium(ii) complexes†
Abstract
To develop metallosurfactant-based fluorescent aggregates, it is important to understand the impact of the head and tail parts on the self-assembly behaviour. Herein, a new series of water soluble, emissive double chain surfactant–ruthenium(II) complexes differing in their head group size and chain length, [Ru(bpy)2(DA)2]Cl2 (1), [Ru(bpy)2(HA)2]Cl2 (2), [Ru(phen)2(DA)2]Cl2 (3), [Ru(phen)2(HA)2]Cl2 (4), where bpy = 2,2′-bipyridyl, phen = 1,10-phenanthroline, DA = dodecylamine and HA = hexadecylamine, has been synthesized and characterized. For the complexes 1–4, hydrophobicity behaviour, critical aggregation concentration (CAC), thermodynamics of the aggregation (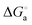 ,
, 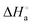 and
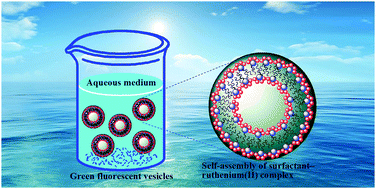
and 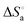 ), and the average size distribution, morphology and stability of the aggregates have been evaluated. The obtained results have shown that the increase in chain length as well as the size of the aromatic head group decreases the CAC values in the order of the complexes 1 > 2 > 3 > 4, whereas those changes increase the hydrophobicity and the average size distribution. The thermodynamics of the aggregation indicated that the process is kinetically controlled, spontaneous, exothermic and entropy driven. The self-assembled surfactant–ruthenium(II) complexes are preferably spherical and belong to the vesicles family being green emissive and monodisperse and showing narrow size distribution and excellent stability in aqueous medium. The enlargement of vesicle size is noted upon increasing the head size as well as the chain length, but the former influences to a greater extent. This type of fluorescent metallovesicles can be used for biomedical and material applications in near future.
), and the average size distribution, morphology and stability of the aggregates have been evaluated. The obtained results have shown that the increase in chain length as well as the size of the aromatic head group decreases the CAC values in the order of the complexes 1 > 2 > 3 > 4, whereas those changes increase the hydrophobicity and the average size distribution. The thermodynamics of the aggregation indicated that the process is kinetically controlled, spontaneous, exothermic and entropy driven. The self-assembled surfactant–ruthenium(II) complexes are preferably spherical and belong to the vesicles family being green emissive and monodisperse and showing narrow size distribution and excellent stability in aqueous medium. The enlargement of vesicle size is noted upon increasing the head size as well as the chain length, but the former influences to a greater extent. This type of fluorescent metallovesicles can be used for biomedical and material applications in near future.


 Please wait while we load your content...
Please wait while we load your content...