Not all third-row elements experience the fluorine gauche effect: β-fluorinated organophosphorus compounds
Abstract
Conformational analyses of β-fluorinated organophosphorus compounds were theoretically carried out to probe the role of a possible fluorine–phosphorus gauche effect in conformer stabilization, specifically using a phosphine, a phosphine oxide, phosphinic and phosphonic acids, and the corresponding anions as model compounds. This hypothesis emerged from recent findings for β-fluorinated sulfoxides, sulfones, and thionium cations, which exhibit the electrostatic gauche effect. Surprisingly, despite bearing a positively charged phosphorus atom, the studied compounds do not show a gauche preference in most gas phase conformers, while little predominance of gauche conformers in solution was calculated only in some cases, due to intramolecular hydrogen bonds. Steric and electrostatic repulsive interactions are invoked as dominating effects rather than electrostatic and/or hyperconjugative gauche effects, since conformational equilibrium in these compounds is better described by a well-balanced competition between anti and gauche conformers. Since the conformational equilibrium is not polarized towards a single conformer, the unusual three-bond coupling constants 3JP,F and 3JP,H can be useful to verify the changes in conformational populations with solvents, as confirmed by NMR calculations.
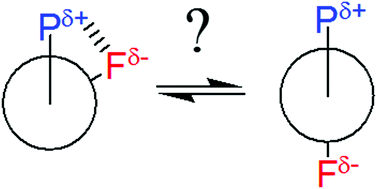


 Please wait while we load your content...
Please wait while we load your content...