Preparation of a macroporous CuAl2O4 spinel monolith and its rapid selective adsorption towards some anionic dyes
Abstract
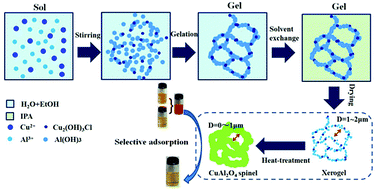
Herein, macroporous CuAl2O4 spinel monoliths derived from ionic precursors were successfully synthesized via a sol–gel route in the presence of propylene oxide (PO) and poly(ethylene oxide) (PEO). PO and PEO cooperatively control the sol–gel transition and phase separation of the system to form gels with a phase-separated macrostructure. The resultant xerogel exists in the form of a monolith and possesses interconnected macroporous channels and co-continuous dense skeletons composed of amorphous Al(OH)3 and Cu2(OH)3Cl. Heat-treatment allows the formation of the single crystalline phase CuAl2O4 spinel without the destruction of well-defined interconnected channels, and the CuAl2O4 spinel monoliths with a macropore size of 0.5–1 μm and porosity of 55–70% can be obtained after heat-treatment. In this study, three anionic dyes and two cationic dyes were selected as adsorbates to investigate the selective adsorption ability of the resultant monoliths. Results indicate that the as-prepared CuAl2O4 spinel heat-treated at 600 °C shows a high affinity and adsorption rate towards the anionic dyes congo red (∼100%) and methyl blue (∼81.8%) within 10 min except towards methyl orange (∼25.5%); thus, the abovementioned spinel can be used for the efficient and selective adsorption of certain anionic dyes from a binary cationic–anionic dye system or fast separation of methyl orange (MO) from a double anionic dye system.


 Please wait while we load your content...
Please wait while we load your content...