Facile one-pot synthesis of 2-amino-1,3,4-oxadiazole tethered peptidomimetics by molecular-iodine-mediated cyclodeselenization†
Abstract
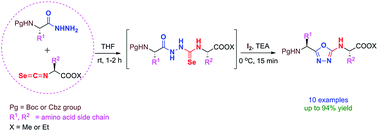
Synthesis of 2-amino-1,3,4-oxadiazole tethered peptidomimetics is described by reaction of Nα-protected amino acid hydrazides with isoselenocyanato esters in one pot. The molecular-iodine-mediated cyclodeselenization of in situ generated selenosemicarbazide intermediates resulted in facile formation of 2-amino-1,3,4-oxadiazoles under mild conditions at excellent yields. The method employs environmentally benign iodine as the cyclizing agent and thereby avoids the harsh conditions employed in existing routes.


 Please wait while we load your content...
Please wait while we load your content...