Redox speciation of uranyl in citrate medium: kinetics and reduction mechanism with in situ spectroelectrochemical investigation†
Abstract
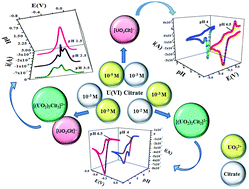
Electrochemical methodology, viz. cyclic and square wave voltammetry, was used to investigate the dominant species of UO22+–citrate at particular physiochemical conditions on the basis of their redox behavior. The UO22+ in citrate media exists as UO22+, monomer [UO2Cit]−, dimer [(UO2)2Cit2]2− and polymeric species. [UO2Cit]− and [(UO2)2Cit2]2− were found to be reduced in two irreversible one-electron steps, with a chemical reaction coupled between them (ICI reduction mechanism), to a stable [UIVCit]+ species at pH ≤ 5 and via a single reduction step with two-electron transfers at pH > 5. Chronopotentiometry and chronoamperometry techniques were applied to corroborate the finding of an ICI reduction mechanism, and to explore the kinetics of reduction by evaluating heterogeneous electron-transfer kinetic (k0f,h and α) parameters. Using the Deford–Hume formula, the stability constant (log β) of monomeric species was calculated to be 6.8 ± 0.11, which is in agreement with the literature. Spectroelectrochemical measurements were carried out to validate the chemical reaction interposed between the two-electron transfer steps. Additionally, evidence of a new U(V) species, i.e., [UVO2Cit]2−, in an aqueous system was obtained by in situ spectroelectrochemical measurements during electrolysis, and its stability constant was obtained by cyclic voltammetry. ESI-MS studies on solutions of varying UO22+ : citrate ratios and pH were used to confirm complex stoichiometries.


 Please wait while we load your content...
Please wait while we load your content...