Synergetic nanoporous Mn–Ru oxides as efficient electrocatalysts for the oxygen reduction reaction†
Abstract
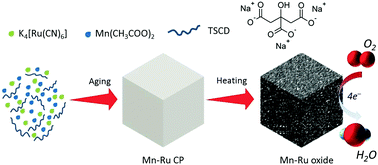
Herein, the rule of using a controlling agent, trisodium citrate dihydrate (TSCD), as a co-reactant in the crystal growth process of Mn–Ru coordination polymer (Mn–Ru CP) nanocubes has been reported. The size and shape of the final product is tuned by increasing the amount of TSCD. Random and aggregated particles were obtained in the absence of TSCD. The shape of the Mn–Ru CP nanocubes was improved using small amounts of TSCD. On further increasing the initial amounts of TSCD, regular and large nanocubes were obtained. In the presence of TSCD, the reaction was slow since the complex appeared to be more stable; thus led to controlled crystal growth. After mixing Mn(CH3COO)2 with TSCD, a Mn-citrate complex was formed. By dropwise addition of a K4[Ru(CN)6] aqueous solution, few Mn2+ ions were slowly released from the complex to react with [Ru(CN)6]4−. Subsequently, the nuclei were generated and further grew to form fine Mn–Ru CP nanocubes. After regular aerobic thermal treatment, synergetic nanoporous Mn–Ru mixed oxides were obtained. The Mn–Ru oxides are promising catalysts for oxygen reduction reactions through an exact four-electron pathway. We speculate that the high catalytic activity of these oxides can be attributed to the fine cubic structure, synergetic composition between Ru and Mn, and intrinsic high electrochemical activity of the ruthenium metal.


 Please wait while we load your content...
Please wait while we load your content...