Click functionalization of poly(glycidyl methacrylate) microspheres with triazole-4-carboxylic acid for the effective adsorption of Pb(ii) ions†
Abstract
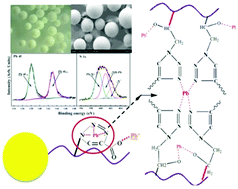
Chelating 1,2,3-triazole-4-carboxylic acids were successfully introduced onto the cross-linked PGMA microsphere surfaces by the combination of ring-opening reactions and the click chemistry process between the alkynyl groups of propiolic acid (PA) and the epoxy groups on the surfaces of the PGMA microspheres. The resultant PA-conjugated PGMA microspheres were found to be an effective adsorbent for Pb(II) removal from aqueous solutions. Success in each functionalization step was ascertained by ATR-FTIR, XPS and SEM characterization. Batch and column adsorption experiments were performed to determine the adsorption kinetics, adsorption isotherms and thermodynamics of Pb(II) ions on the as-synthesized microspheres. The Langmuir-fitted maximum adsorption capacity for Pb(II) ions was found to be 69.41 mg g−1 at a solution pH of 5.0, together with the adsorption equilibrium being achieved within 60 min. The thermodynamic studies demonstrated an endothermic and spontaneous adsorption process of Pb(II) ions on the PA-conjugated PGMA microspheres. Column adsorption studies revealed the decrease in the adsorption capacities of the breakthrough and exhaustion points with increasing flow rate. The postulated adsorption mechanism was attributed to the electrostatic interactions from carboxylic groups and chelation or coordination of Pb(II) ions from the triazole moieties, which was facilitated to form a pluralistic potential trap for Pb(II) ions, as confirmed by XPS characterization.


 Please wait while we load your content...
Please wait while we load your content...