Microwave synthesis of bis(cycloalkeno)-1,4-diselenins: a novel source of Se for CdSe QDs†
Abstract
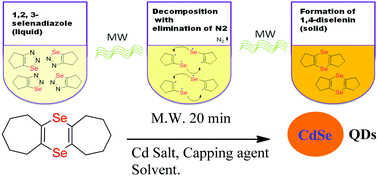
This paper describes the formation of a series of bis(cycloalkeno)-1,4-diselenins from the corresponding cycloalkeno-1,2,3-selenadiazoles by a microwave irradiation (MW) method. The bi-radical dimerization reaction of 1,2,3-selenadiazoles was performed by a new synthetic strategy under solvent-free conditions using 100 Watt microwave irradiation for about 20 minutes. The current synthesis afforded a feasible approach for the preparation of various 1,4-diselenins. The so-prepared alkyl selenides were characterized by various spectroscopic tools, viz. UV-visible, FTIR, 1H, 13C, DEPT and 77Se NMR spectroscopy, ESI-MS and TGA analysis. Additionally, bis(cyclohepteno)-1,4-diselenin has been effectively utilised as a novel selenium precursor for the synthesis of CdSe quantum dots (QDs). This Se-rich precursor can likely be used for the preparation of other metal selenides also.


 Please wait while we load your content...
Please wait while we load your content...