Facile synthesis of highly thermally stable TiO2 photocatalysts
Abstract
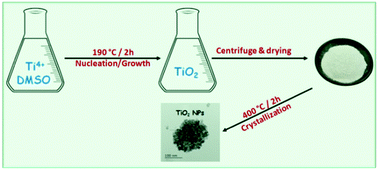
A new and facile method of synthesizing high-temperature stable titanium dioxide (TiO2) nanoparticles (NPs) is presented in this work. This novel approach allows the production of titanium dioxide nanoparticles owing to a modified solvothermal process that makes use of titanium(IV) butoxide as a titanium precursor and Dimethyl Sulfoxide (DMSO) as a solvent. The structure and morphology of the TiO2 nanoparticles were characterized by X-ray diffraction (XRD), transmission electron microscopy (TEM), energy dispersive X-ray spectrometry (EDX) and high-resolution transmission electron microscopy (HRTEM). Based on FTIR and TDA/TGA measurements, a proposed mechanism for the formation of TiO2 NPs in DMSO (without adding any other reagents) is discussed in this contribution. Optical absorption measurements showed that the TiO2 nanoparticles exhibited a UV significant absorption peak clearly blue-shifted with respect to that of bulk TiO2. The results showed that monodisperse quasi-spherical TiO2 nanoparticles (with an average size of 11 nm) consisting of a pure anatase phase were formed. The titanium dioxide nanoparticles showed a high photocatalytic performance in the degradation of diuron pesticide (C9H10Cl2N2O) under illumination by UV light. The high crystalline quality, together with the easy synthesis process, makes TiO2 nanoparticles a promising candidate for many applications, such as optoelectronics and water photolysis for hydrogen production.


 Please wait while we load your content...
Please wait while we load your content...