Aza boron-pyridyl-isoindoline analogues: synthesis and photophysical properties†
Abstract
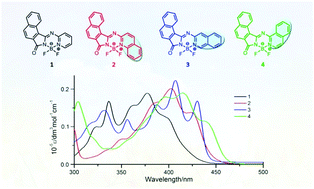
Several aza boron-pyridyl-isoindoline analogues are synthesized through a facile and scale-up two step reaction using 1,2-naphthalenedicarbonitrile as a starting material. These analogues show broad envelopes of intense vibrational bands in the absorption spectra with moderate fluorescence quantum yields in solution and the solid-state. An analysis of the structure–property relationships is described based on X-ray crystallography, optical spectroscopy, and theoretical calculations.


 Please wait while we load your content...
Please wait while we load your content...