Polarity of tetraalkylammonium-based ionic liquids and related low temperature molten salts†
Abstract
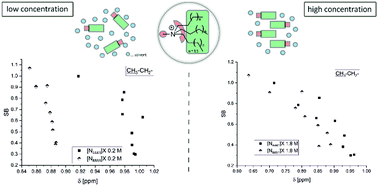
Solvatochromic measurements and 1H NMR spectroscopy have been used to investigate the overall polarity of tetraalkylammonium (R3N+–CH3 with R = n-C4H9; n-C8H17) based ionic liquids (IL) and low temperature molten salts (LTMS), respectively, as function of the counter anion. Solvent acidity (SA), solvent basicity (SB), polarizability (SP) and dipolarity (SdP) parameters have been determined according to the Catalán scale. [FeII(1,10-phenanthroline)2(CN)2] (Fe), 3-(4-amino-3-methylphenyl)-7-phenylbenzol[1,2-b:4,5-b′]difuran-2,6-dione (ABF), 3-(4-N,N-dimethylaminophenyl)-7-phenylbenzol[1,2-b:4,5-b′]-difuran-2,6-dione (DMe-ABF), 4-tert-butyl-2-(dicyanomethylene)-5-[4-(dimethylamino)benzylidene]-Δ3-thiazoline (Th), and 2-[4-(N,N-dimethylamino)benzylidene]malononitrile (BMN) have been used as solvatochromic polarity indicators. Significant correlations of the SB parameter of anion X− as a function of the chemical shift of the H-atoms of R3N+–CH3X− are presented and discussed. Thus, IL solvent basicity (SB) of the anion determines the interaction strength towards those CH-bonds in the direct vicinity of the ammonium nitrogen atom.


 Please wait while we load your content...
Please wait while we load your content...