Electrocatalysts composed of a Co(acetylacetonate)2 molecule and refluxed graphene oxide for an oxygen reduction reaction†
Abstract
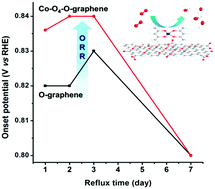
Hybridization of organometallic complexes with carbon-based materials has been shown to enhance the catalytic performance in the oxygen reduction reaction (ORR). As chemical interactions between the metal centers and ligands are critical factors determining their tunable catalytic nature, it is important to understand the correlation between the chemical structure and catalytic nature and the correlation between the chemical structure and catalytic performance of the hybrids. In this work, the hybrids are synthesized by the reaction of an organometallic complex, Co(II)(acac)2 (acac = acetylacetonate), with refluxed graphene oxide (Re-G-O) materials containing controlled amounts of oxygen atoms at room temperature. Experimental characterization of the hybrids reveals that Co(II)(acac)2 is coordinated to oxygen containing groups in Re-G-O, generating organometallic species, Co–O4–O. These hybrids showed better electrocatalytic activity for the ORR in alkaline media than Co-free Re-G-O materials. The results show that the Co–O4 species is catalytically active for the ORR. We find that the best Co–O4 catalyst for the ORR is produced by the use of reduced graphene oxide with a medium level of reduction.


 Please wait while we load your content...
Please wait while we load your content...