Hydrogenation of 3-nitro-4-methoxy-acetylaniline with H2 to 3-amino-4-methoxy-acetylaniline catalyzed by bimetallic copper/nickel nanoparticles†
Abstract
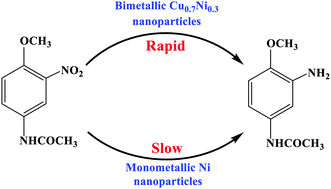
Monometallic Cu, monometallic Ni, and bimetallic CuxNiy nanoparticles were prepared by a wet chemical reduction method. A Cu–Ni alloy phase was formed in the bimetallic CuxNiy nanoparticles. The bimetallic CuxNiy nanoparticles exhibited higher catalytic activity for the hydrogenation of 3-nitro-4-methoxy-acetylaniline (NMA) to 3-amino-4-methoxy-acetylaniline (AMA) with H2 than the sole Ni nanoparticles, which was ascribed to the effect of the Cu–Ni alloy phase. When the reaction was catalyzed over the bimetallic Cu0.7Ni0.3 catalyst at 140 °C for 2 h, the AMA selectivity was 99.4% when the NMA conversion was 95.7%. The reaction activation energy for NMA hydrogenation was 19.74 kJ mol−1.


 Please wait while we load your content...
Please wait while we load your content...