An improved quantum biochemistry description of the glutamate–GluA2 receptor binding within an inhomogeneous dielectric function framework†
Abstract
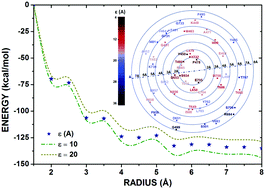
Glutamatergic receptors are prominent targets for the treatment of several neurological disorders like epilepsy, amyotrophic lateral sclerosis, and Parkinson's disease. Hence an improved understanding of how glutamate interacts with the ligand binding domain of these receptors can bring crucial insights into the development of new ligands for this system. In this work, a DFT study on the GluA2–glutamate interaction was carried out using the structure of GluA2 co-crystallized with the glutamate ligand. We developed a novel model to describe the non-uniform dielectric function of the GluA2 receptor, evaluating the binding energy of the GluA2–glutamate system accordingly and comparing the results with those obtained using a fixed dielectric function. Our study exhibited significant correlation with experimental data and previous calculations, allowing us to estimate an optimal choice for the dielectric function to simulate the GluA2–ligand interaction.


 Please wait while we load your content...
Please wait while we load your content...