Efficient phenanthroimidazole-styryl-triphenylamine derivatives for blue OLEDs: a combined experimental and theoretical study†
Abstract
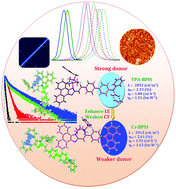
Blue emitting devices based on N-(4-(4′-(1-(4-benzylpiperidine)-1H-diphenylimidazol-2-yl)-styryl-1)phenyl)-N-phenylbenzenamine (TPA-BDIS), N-(4-(4′-(1-(4-benzylpiperidine)-1H-phenanthro[9,10-d]imidazol-2-yl)-styryl-1)phenylbenzenamine (TPA-BPIS) and 2-(4′-9H-carbazol-9-yl)-[1,1′-styryl]-4-yl-1-benzylpiperidine-1H-phenanthro[9,10-d]imidazole (Cz-BPIS) have been synthesized and characterized. The fully twisted TPA-BDIS exhibits a narrow electroluminescence emission at 441 nm with a full-width at half maximum of 40 nm. Density functional theory (DFT) calculations show that the decreased energy barrier between the transitions of TPA-BDIS causes a strong orbital coupling resulting in a deep blue emission. The electroluminescence performance of the non-doped device based on TPA-BPIS shows a high brightness of 2852 cd m−2, a current efficiency of 1.88 cd A−1, a power efficiency of 1.51 lm W−1 and an external quantum efficiency of 2.53%. Compared with TPA-BPIS, the weak donor carbazole substituted Cz-BPIS exhibits an excellent performance: a blue emission with CIE coordinates of (0.16, 0.09), a current density of 1.91 cd A−1, a power efficiency of 1.63 lm W−1 and an external quantum efficiency of 2.61%. A fluorescent molecule with a combination of the HLCT state and hot excitons may be an ideal candidate in the design of high efficiency, low cost, fluorescent OLED materials.


 Please wait while we load your content...
Please wait while we load your content...