Sonogashira coupling in 3D-printed NMR cuvettes: synthesis and properties of arylnaphthylalkynes†
Abstract
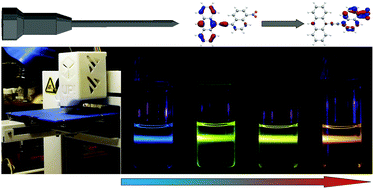
3D-Printing has been used to build hollow NMR tube/spinner combinations out of NMR-transparent polyamide. The whole 3D-print has been processed inside a glove box and during pauses of the 3D-printing process, all reactants for the palladium-catalyzed decarboxylative Sonogashira coupling of aryl halides with arylpropiolic acids have been inserted. Within the totally gas tight and pressure resistant tubes, a set of arylnaphthylalkynes has been synthesized and the progress of the reaction has been monitored via NMR spectroscopy. The (nonlinear) optical properties of the push–pull substituted bis(aryl)alkynes have been investigated by fluorescence spectroscopy and DFT calculations. A significant correlation between the intensity of the triple-bond stretching vibration and the calculated first hyperpolarizability is reported.


 Please wait while we load your content...
Please wait while we load your content...