Synthesis, photophysical and concentration-dependent tunable lasing behavior of 2,6-diacetylenyl-functionalized BODIPY dyes†
Abstract
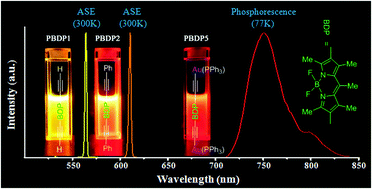
2,6-Diacetylenyl- and 2,6-bis(arylacetylenyl)-functionalized pentamethyl-difluoroborondipyrromethene (BODIPY) derivatives, namely, PBDP1 and PBDP2–4 (aryl = phenyl, 4-methoxyphenyl, or 4-cyanophenyl), respectively, which exhibit extended π-conjugation, were synthesized and characterized by various spectroscopic methods. Significant bathochromic shifts in both absorption and emission were observed upon modifying the structure of the BODIPY core via the strategy of extending its π-conjugation. The derivatives displayed efficient emission in the yellow-to-red spectral region, with a high fluorescence quantum yield and a relatively large Stokes shift. Under conditions of transverse pumping in a cuvette, PBDP1 and PBDP2 exhibited highly efficient and stable laser activity for up to 180 and 110 minutes of continuous irradiation, respectively. Amplified spontaneous emission (FWHM of ca. 2.5 nm) with an efficiency of 41% and 36% was achieved for PBDP1 and PBDP2, respectively, in toluene, which had tunable ranges of 561 to 580 nm and 602 to 617 nm, respectively, on irradiation with a Q-switched Nd:YAG laser at 532 nm. The lasing properties of PBDP3 and PBDP4, which contain electron-donating (–OMe) and electron-withdrawing (–CN) arylacetylenyl moieties, respectively, were also investigated. A corresponding digold(I) diacetylide organometallic complex, namely, (PPh3)Au–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–BODIPY–C
C–BODIPY–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–Au(PPh3) (PBDP5) was also synthesized and characterized to study the effect of Au(I). PBDP5 exhibited phosphorescence in the vis-NIR region centered at 751 nm at 77 K owing to heavy-atom-induced intersystem crossing.
C–Au(PPh3) (PBDP5) was also synthesized and characterized to study the effect of Au(I). PBDP5 exhibited phosphorescence in the vis-NIR region centered at 751 nm at 77 K owing to heavy-atom-induced intersystem crossing.


 Please wait while we load your content...
Please wait while we load your content...