Alkyne–azide cycloaddition analogues of dehydrozingerone as potential anti-prostate cancer inhibitors via the PI3K/Akt/NF-kB pathway†
Abstract
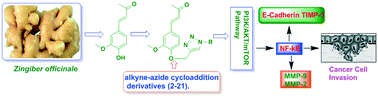
Herein, we report the isolation and synthetic modification of dehydrozingerone (DHZ, 1), a secondary metabolite present in the rhizome of Zingiber officinale. We synthesized O-propargylated dehydrozingerone, which was subsequently coupled by alkyne–azide cycloaddition (3–20) using click chemistry. The compounds (1–20) were evaluated for their in vitro cytotoxic activity in a panel of three cancer cell lines. Among all the DHZ derivatives, 3, 6, 7, 8, 9 and 15 displayed potent cytotoxic potential with an IC50 value ranging from 1.8–3.0 μM in MCF-7, PC-3 and HCT-116 cell lines. Furthermore, compound 7 has proven to be the most potent cytotoxic compound in all the three distinct cancer cell lines and also demonstrated significant anti-invasive potential in prostate cancer. The mechanistic study of compound 7 showed that it not only suppressed the AKT/mTOR signalling which regulates nuclear transcription factor-NF-kB but also augmented the expression of anti-invasive markers E-cadherin and TIMP. Compound 7 significantly decreased the expression of pro-invasive markers vimentin, MMP-2 and MMP-9, respectively. This study underscores an efficient synthetic approach employed to evaluate the structure–activity relationship of dehydrozingerone (1) in search of potential new anticancer agents.


 Please wait while we load your content...
Please wait while we load your content...