Development of novel β-carboline-based hydroxamate derivatives as HDAC inhibitors with DNA damage and apoptosis inducing abilities†‡
Abstract
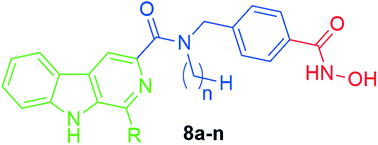
A series of novel β-carboline-based hydroxamate derivatives (8a–n) as HDAC inhibitors have been designed and synthesized. Most of these compounds displayed potent histone deacetylase inhibitory effects and good antiproliferative activity with IC50s in the low micromolar range. One of the most potent compounds (8k) showed the strongest inhibition of the proliferation of human hepatocellular carcinoma (HCC) cells in vitro, with IC50 values lower than that of the currently approved HDAC inhibitor SAHA. Compound 8k also increased acetylation of histone H3 and α-tubulin, consistent with its potent HDAC inhibition. Importantly, 8k induced hypochromism by electrostatic interactions with CT-DNA, suggesting potential induction of DNA damage. Finally, 8k significantly induced HepG2 cell apoptosis by regulating apoptotic relative proteins expression. Together, our findings suggest that these novel β-carboline-based hydroxamate derivatives may provide a new framework for the discovery of novel antitumor agents for the intervention of human carcinoma cells.


 Please wait while we load your content...
Please wait while we load your content...