Capillary-based integrated digital PCR in picoliter droplets†
Abstract
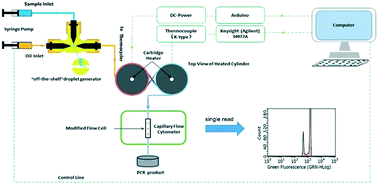
The droplet digital polymerase chain reaction (ddPCR) is becoming more and more popular in diagnostic applications in academia and industry. In commercially available ddPCR systems, after they have been made by a generator, the droplets have to be transferred manually to modules for amplification and detection. In practice, some of the droplets (∼10%) are lost during manual transfer, leading to underestimation of the targets. In addition, the droplets are also at risk of cross-contamination during transfer. By contrast, in labs, some chip-based ddPCRs have been demonstrated where droplets always run in channels. However, the droplets easily coalesce to large ones in chips due to wall wetting as well as thermal oscillation. The loss of droplets becomes serious when such ddPCRs are applied to absolutely quantify rare mutations, such as in early diagnostics in clinical research or when measuring biological diversity at the cell level. Here, we propose a capillary-based integrated ddPCR system that is used for the first time to realize absolute quantification in this way. In this system, a HPLC T-junction is used to generate droplets and a long HPLC capillary connects the generator with both a capillary-based thermocycler and a capillary-based cytometer. The performance of the system is validated by absolute quantification of a gene specific to lung cancer (LunX). The results show that this system has very good linearity (0.9988) at concentrations ranging from NTC to 2.4 × 10−4 copies per μL. As compared to qPCR, the all-in-one scheme is superior both in terms of the detection limit and the smaller fold changes measurement. The system of ddPCR might provide a powerful approach for clinical or academic applications where rare events are mostly considered.


 Please wait while we load your content...
Please wait while we load your content...