Photoelectrochemical ion concentration polarization: membraneless ion filtration based on light-driven electrochemical reactions†
Abstract
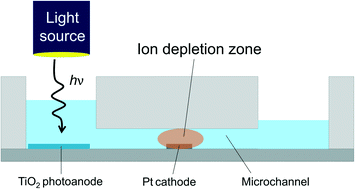
In this article we report a microelectrochemical system that is able to partially desalinate water. The underlying principles are similar to previous reports in which a local electric field resists passage of ions. However, in the present case, no membrane is required and, most interestingly, much of the power for desalination originates from light rather than electricity. This could greatly increase the power efficiency for desalination. The device is based on a TiO2 photoanode coupled to a Pt cathode. Illumination of the photoanode drives faradaic reactions at the cathode that lead to an ion depletion zone. The resulting local electric field limits transport of charged species. In situ conductivity and fluorescence measurements demonstrate the effectiveness of the device.


 Please wait while we load your content...
Please wait while we load your content...