Highly selective sp3 C–N bond activation of tertiary anilines modulated by steric and thermodynamic factors†
Abstract
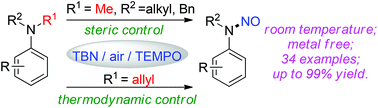
A highly selective sp3 C–N cleavage of tertiary anilines was achieved using the TBN/TEMPO catalyst system. When N,N-diaklylanilines (alkyl, benzyl) were employed, the N–CH3 bond was selectively cleaved via radical C–H activation. Moreover, when the allyl group was installed, totally reverse selectivity was observed. It is worth noting that the solvent effect is also crucial to obtain high reaction efficiency and selectivity.


 Please wait while we load your content...
Please wait while we load your content...