Insight into forced hydrogen re-arrangement and altered reaction pathways in a protocol for CO2 catalytic processing of oleic acid into C8–C15 alkanes†
Abstract
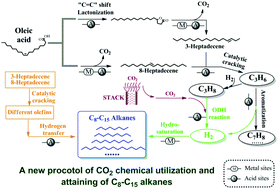
A new vision of using carbon dioxide (CO2) catalytic processing of oleic acid into C8–C15 alkanes over a nano-nickel/zeolite catalyst is reported in this paper. The inherent and essential reasons which make this achievable are clearly resolved by using totally new catalytic reaction pathways of oleic acid transformation in a CO2 atmosphere. The yield of C8–C15 ingredients reaches 73.10 mol% in a CO2 atmosphere, which is much higher than the 49.67 mol% yield obtained in a hydrogen (H2) atmosphere. In the absence of an external H2 source, products which are similar to aviation fuel are generated where aromatization of propene (C3H6) oxidative dehydrogenation (ODH) involving CO2 and propane (C3H8) and hydrogen transfer reactions are found to account for hydrogen liberation in oleic acid and achieve its re-arrangement in the final alkane products. The reaction pathway in the CO2 atmosphere is significantly different from that in the H2 atmosphere, as shown by the presence of 8-heptadecene, γ-stearolactone, and 3-heptadecene as reaction intermediates, as well as a CO formation pathway. Because of the highly dispersed Ni metal center on the zeolite support, H2 spillover is observed in the H2 atmosphere, which inhibits the production of short-chain alkanes and reveals the inherent disadvantage of using H2. The CO2 processing of oleic acid described in this paper will significantly contribute to future CO2 utilization chemistry and provide an economical and promising approach for the production of sustainable alkane products which are similar to aviation fuel.


 Please wait while we load your content...
Please wait while we load your content...