A highly efficient Suzuki–Miyaura methylation of pyridines leading to the drug pirfenidone and its CD3 version (SD-560)†
Abstract
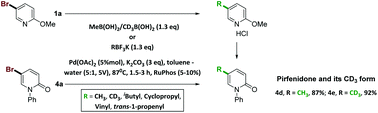
Efficient introduction of methyl or methyl-d3 into aromatic and heteroaromatic systems still presents a synthetic challenge. In particular, we were in search of a non-cryogenic synthesis of the 5-CD3 version of pirfenidone (4d, also known as Pirespa®, Esbriet® or Pirfenex®), one of the two drugs approved to date for retarding idiopathic pulmonary fibrosis (IPF), a serious, rare and fatal lung disease, with a life expectancy of 3–5 years. The methyl-deuterated version of pirfenidone (4e, also known as SD-560) was designed with the objective of attenuating the rate of drug metabolism, and our goal was to find a green methylation route to avoid the environmental and economic impact of employing alkyllithium at cryogenic temperatures. The examination of several cross-coupling strategies for the introduction of methyl or methyl-d3 into methoxypyridine and pyridone systems culminated in two green and nearly quantitative Suzuki–Miyaura cross-coupling routes in the presence of RuPhos ligand: the first, using commercially available methyl boronic acid or its CD3 analog and the second, employing potassium methyl trifluoroborate or CD3BF3K, the latter obtained by a new route in 88% yield. This led, on a scale of tens of grams, to the synthesis of pirfenidone (4d) and its d3 analog, SD-560 (4e), at 99% isotopic purity.


 Please wait while we load your content...
Please wait while we load your content...