A continuous flow process for the production of 2,5-dimethylfuran from fructose using (non-noble metal based) heterogeneous catalysis†
Abstract
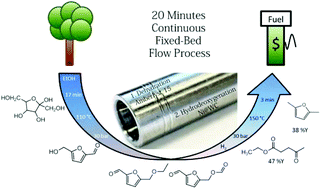
The abundant carbohydrate fructose is converted into two biofuel molecules, namely 2,5-dimethylfuran (DMF) and ethyl levulinate (EL) in a simple cascade flow reactor. With an overall yield of 85% (38.5% of 2,5-dimethylfuran and 47% of ethyl levulinate), the main remainder is unconverted fructose. The two column flow reactor set-up enables the adjustment of temperatures and reaction times in such a way that the reactive intermediate hydroxymethylfurfural (5-HMF) is generated in optimal yields and converted into the stable DMF immediately. The process is so simple and fast (<20 min) that economic and sustainable production of these fuels and platform chemicals can be envisioned. A remaining minor char formation is regarded to be the major problem which has to be addressed by catalyst development.


 Please wait while we load your content...
Please wait while we load your content...