Metal-free reduction of the greenhouse gas sulfur hexafluoride, formation of SF5 containing ion pairs and the application in fluorinations†
Abstract
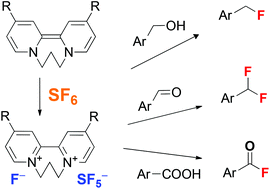
A protocol for the fast and selective two-electron reduction of the potent greenhouse gas sulfur hexafluoride (SF6) by organic electron donors at ambient temperature has been developed. The reaction yields solid ion pairs consisting of donor dications and SF5-anions which can be effectively used in fluorination reactions.


 Please wait while we load your content...
Please wait while we load your content...