Phase-out-compliant fluorosurfactants: unique methimazolium derivatives including room temperature ionic liquids†
Abstract
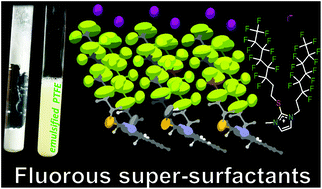
Expedient alkylations of 1-methyl-3H-imidazole-2-thione, a pharmaceutical active ingredient available in bulk quantities, provide high yield access to numerous protonated, or quaternized imidazoliums with chemospecific attachment of the fluoroponytail at the 2-mercapto functionality. The deprotonated primary target products represent valuable nitrogen heterocyclic bases, capable of further substitution, and salt or complex formation. Specific physicochemical characteristics that are relevant for phase and surface responsive behavior, e.g. critical micelle concentration, oleophobicity, depression of the aqueous surface tension, foam formation, emulsification of microgranular PTFE, are investigated for selected representatives and compared to the properties of common fluorosurfactants and ionic liquids. Remarkably, it is found that halogen bonding between iodide counterions of respective polyfluoroalkylmethimazolium systems and 1-iodoperfluoroalkanes, serving as the σ-hole partner of the halides, greatly affect the solubility profile of the resulting molecular adducts. Single-crystal X-ray structure determinations are carried out across the new fluorous substance classes. Strikingly, the helical arrangement of fluorine atoms along the chains typically encountered in polyfluorinated compounds is not found as the prevailing conformation. Rather, they are outnumbered by structural motifs exhibiting the rare zig-zag (linear alkane-like) chain conformation. Key derivatives are also subjected to preliminary ecotoxicological testing.
- This article is part of the themed collection: 2017 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...