A hydroaminomethylation/hydrohydroxymethylation sequence for the one pot synthesis of aminohydroxytriglycerides†
Abstract
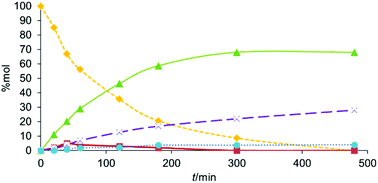
In this paper we report the one pot functionalization of the carbon–carbon double bonds of triglycerides via a cascade hydroaminomethylation (HAM)/hydrohydroxymethylation (HHM) reaction. The carbon–carbon double bonds are first hydroformylated using a Rh-catalyst. The produced aldehydes react with secondary amines to yield enamines which are then reduced to tertiary amines. Interestingly, the latter act as ligands of Rh-species capable of hydrogenating the remaining formyl groups into alcohols. As a result, the final triglycerides are substituted by both aminomethyl and hydroxymethyl groups. Of interest, no phosphane ligand is used to stabilize the Rh-species. Moreover, the proportion of aminomethyl and hydroxymethyl groups grafted onto the triglyceride fatty chains can be finely adjusted by a careful choice of the experimental conditions, especially the nature and the amount of the amine, the reaction temperature, and the CO/H2 pressure. The resulting amino-hydroxylated triglycerides are unprecedented biosourced building blocks whose applications could be of major interest, especially in the field of polymer chemistry.


 Please wait while we load your content...
Please wait while we load your content...